Talk Overview
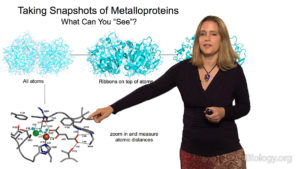
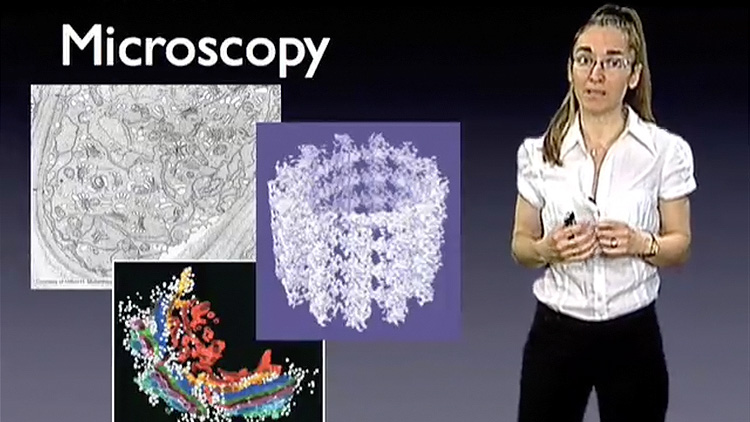
Dr. Drennan begins her lecture by explaining what metalloproteins are and how the inclusion of a metal in the protein structure results in amazingly reactive proteins. She describes techniques, such as X-ray crystallography and electron microscopy, which her group uses to get “snapshots” of metalloproteins in action. These “snapshots” allow them to better understand how metalloproteins function in many critical biological reactions.
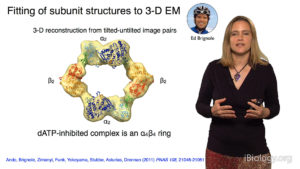
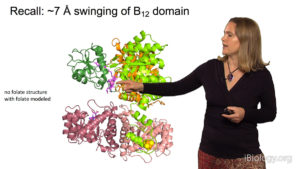
In Part 2, Drennan focuses on experiments her lab has done to understand the mechanism of action of ribonucleotide reductase (RNR), a key enzyme required for DNA synthesis and repair and, consequently, cell viability. By taking “snapshots” of bacterial RNR, Drennan and her colleagues deciphered how changes in the structure of RNR regulated its activity. Interestingly, the structure of bacterial RNR appears to differ from that of human RNR suggesting a possible new target for antibiotics.
In the last part of her talk, Drennan explains how cobalt based metalloproteins allow acetogenic bacteria to live on CO2. This group of bacteria convert more than 1010 tons of CO2 to acetate each year and scientists are interested in understanding and using these metalloproteins to remove CO2 from the atmosphere. Structures determined by Drennan’s lab have provided information on the mechanism of action of these important enzymes.
Speaker Bio
Catherine Drennan

Cathy Drennan is a Professor of Chemistry and Biology at the Massachusetts Institute of Technology and a Professor and Investigator of the Howard Hughes Medical Institute. She studied chemistry as an undergraduate at Vassar College, received her PhD in biological chemistry from the University of Michigan, was a post-doc at the California Institute of Technology… Continue Reading







Leave a Reply