Dr. Koen Van Rompay is a virologist at the California National Primate Research Center (CNPRC) at UC Davis with expertise in nonhuman primate models of viral infections. He has worked extensively on HIV and Zika virus, and is now part of a cooperative effort to develop a coronavirus vaccine. He was on the forefront of HIV treatment research at the CNPRC in the 1990s, helping to develop and test the anti-viral drug tenofovir, now the key ingredient in pre- and post-exposure prophylaxis regimens (“PrEP”) to prevent transmission of HIV. Dr. Van Rompay was featured in our iBiology video about coronaviruses and infectious disease outbreaks, Studying Coronaviruses: Vectors to Vaccines. So much of our conversation went beyond what we could include in the video, but here are a few more of the answers he offered to our questions. Questions and responses have been edited for clarity.

Can you explain how animal models play a role in the development of vaccines and treatments for disease?
To study diseases and also to cope with interventions, it’s often very difficult to get all the information we need from studying humans. That’s why ideally, we want to study diseases in animal models that mimic the human disease. There are many different animal models that actually range from, for example, rats and mice, to dogs, and also monkeys. So often, when you want to test certain interventions, the first step is usually to show that some compound or something works in vitro in the test tube. But of course, it’s a very big difference on what you test in vitro and how our human body works. So that’s why usually when something looks promising in vitro, you want to test it in animal models.
So if the compound or molecule you are testiing is able to inhibit the virus and it doesn’t kill the cells, that’s kind of good evidence to suggest moving into studies using animal models using mice or rats, for example. And from there we proceed down what we call the preclinical pipeline, where we move from mice and rats to a higher species eventually, then monkeys. And then if that’s then really looks good, we can then move that into human clinical trials.
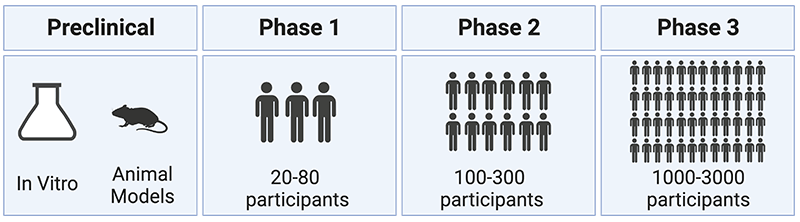
Once you get to that stage, what is the clinical trial process like?
The process to develop a vaccine and get it approved is very long and quite tedious. You first want to know what the virus is. Once you know that, you want to see which part of the virus should I use in the vaccine construct to induce the proper immune response to give protection. Because if you give a vaccine depending on which protein you use, you can induce different immune responses. Some immune responses may be protective, some of them may be ineffective in very rare cases, and immune responses can actually be harmful. So of course, you really want to then test those vaccines in animal models to look both for efficacy and for safety. Safety is always a very important concern in vaccine development, because if you want to roll out a vaccine on a large scale, you want to make sure that you don’t do any harm.
And so, that whole process of developing the vaccine construct, testing it in animal models, and then moving into human trials are done in different stages. Usually, you have a phase one trial where you test it on a very limited number of people to see if it is safe. And if it induces the desired immune response. If that looks promising then you go to phase two, where you test it in more people, and then if that still looks promising, then you will go to phase three trial. And the phase three trial is kind of the first one where you really want to look at whether the vaccine really works. Does it protect against infection?
Often to do phase three clinical trials, you need very large numbers of people. To be able to do a placebo controlled trial, you want to have an active site where you have an epidemic that’s happening there, right now, where you can expect that enough people get exposed to the virus to become infected. So, you need to have all those things in place and let’s write it can often be very long, very tedious to actually bring a vaccine on the market. With some outbreaks, for example, in the early 2000s, the SARS epidemic had already faded away by the time people actually had a possible vaccine candidate. So there wasn’t really any active outbreak happening anymore to be able to test that vaccine in the phase three human trial.
It seems like the timeline for developing a vaccine can be quite long. How does this work when there is such urgent need during an active outbreak?
Yeah, exactly. So the time is very long. Sometimes we can be lucky if the virus is in a family in which other viruses have already caused outbreaks beforehand. If there are approved vaccines or drugs already on the market, they may give some partial protection against a new outbreak. We can actually use that to our benefit because then we have something that has already been approved to be effective and safe. Even if it only works partially against a new emerging disease, it’s a lot easier to roll it out, to give it to people as a vaccine to protect them against possible infection. Or to even give it to people who were already infected and who already are showing some symptoms. So that’s actually now also happening with the new human coronavirus. There are some of those studies happening with drugs that have already been tested before against other viruses from the same family.
In later communication with Dr. Van Rompay, we clarified that the work he is doing is focused on the development of vaccines using new, untested compounds. If you have seen information in the news about vaccine developments for COVID-19 in various stages of clinical trials, these are compounds that have previously been approved for other diseases but may be effective for COVID-19.

What is the treatment and care like for animals in the facilities where you do your research?
So when we want to do research on nonhuman primates, we really provide the best optimal care to these animals. We have a whole team of people who know how to provide the best kind of husbandry to these animals, the best kind of food and nutrition. Also, when animals get sick they get very high quality veterinary care. We want the animals to be as healthy as possible. When we do research on those animals, for example when we have to infect them with certain agents, we also give them optimal supportive care.
That sort of gives me comfort that the research that we do, we minimize the suffering of our animals. And that sort of tells me that this is really worth doing. We take care of the animals very well, we are able to get very useful data out of this. The research that we do benefits humans, but also benefits animals. Most of us have pets at home, and also those pets have benefitted from much research that has been done in laboratory animals, whether it’s development of vaccines against diseases that can affect our pets.





Craig A. Brookins says
Thank you for your commitment to research and dedication to treating disease. I am a Newfoundland dog owner and I know that the Newfoundland Club of America sponsors much basic canine disease research. I agree that we must continue funding not only viral research but also research on diseases from all forms of pathogens. I believe this is a unique time to push this message forward.
Amanda M. Dettmer, PhD says
Thank you, Dr. Van Rompay, for you important work not only in vaccine development, but also in communicating about this work —and especially the irreplaceable value of animal models and how we in the research community care for them.