Talk Overview
Dr. Spudich begins his talks with a clear overview of muscle biology. Muscles are made of many cells and each cell contains many contractile units called sarcomeres. Sarcomeres are made of parallel filaments of two different proteins, actin and myosin. The filaments slide relative to each other and cause the sarcomere, and in turn the muscle, to contract. Spudich recounts the breakthroughs that led muscle biologists to propose this sliding filament model (~1950). By 1969, experiments using electron microscopy, a relatively new technology at the time, and X-ray diffraction, had advanced the model to explain how the “swing” of individual myosin molecules along actin filaments could power muscle contraction.
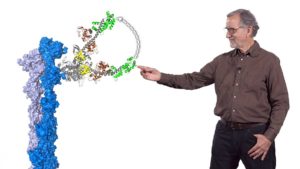
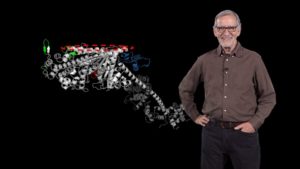
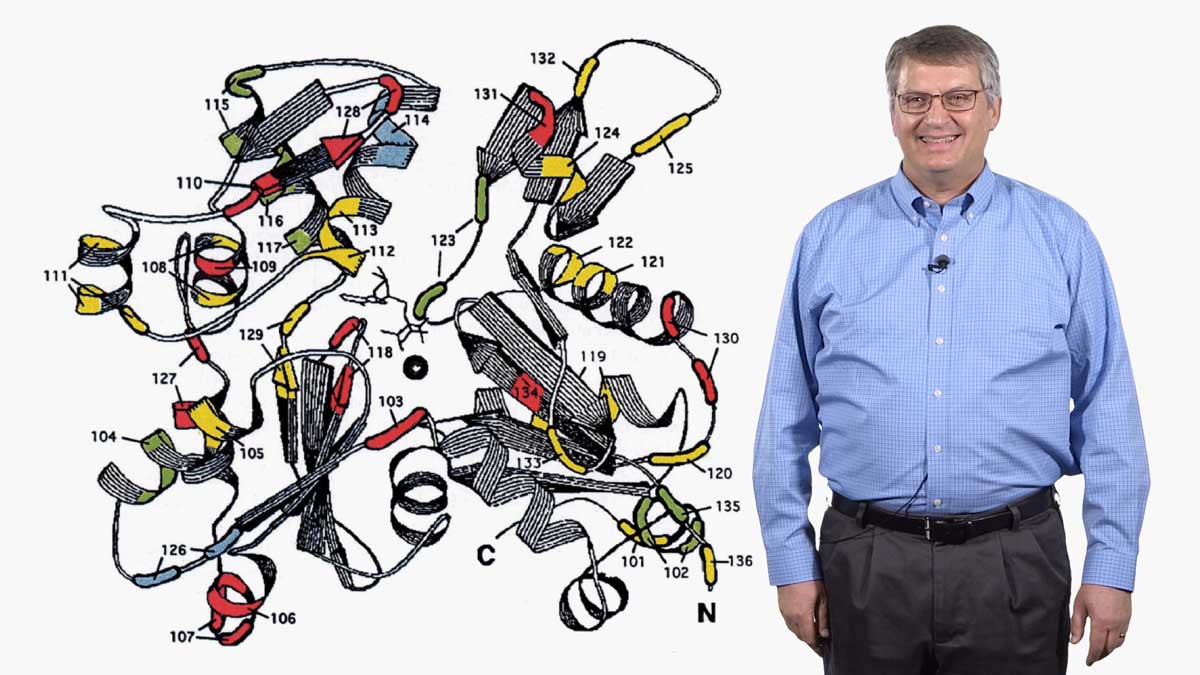
In his second talk, Spudich describes the technological and experimental advances of the last ~50 years that have allowed researchers to understand muscle contraction in molecular detail. The development of an in vitro assay let Spudich and his colleagues determine which domain of the myosin molecule (which is very large) is necessary for movement. A laser trap assay allowed them to measure the size of the “step” taken by a myosin molecule moving along actin, as well as the force generated by a single myosin molecule. The solution of a crystal structure for myosin also provided key information about its mechanism of action. These results, together with early enzymatic analysis, have led to a detailed molecular model for the chemo-mechanical cycle of myosin.
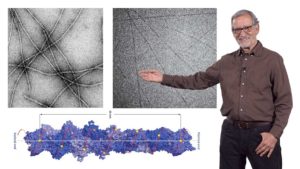
In his third talk, Spudich recounts his first foray into muscle research as a postdoc in Hugh Huxley’s lab. He wanted to understand how Ca2+ regulated muscle contraction via the troponin/tropomyosin complex. Using electron micrographs and diffraction analysis to investigate where tropomyosin filaments lie along actin filaments, Spudich showed that tropomyosin filaments block the myosin-binding sites on actin. When muscle is stimulated, Ca2+ is released from intracellular stores and binds to troponin causing tropomyosin to move. This allows myosin to bind to actin and the muscle to contract. These finding led Spudich and Huxley to propose a steric blocking mechanism for regulation of muscle contraction. Recent technological improvements have confirmed this model and filled in important details.
In his last talk, Spudich focuses on current studies in his lab to understand how mutations in cardiac myosin cause human hypertrophic cardiomyopathy (HCM). This is a disease characterized by a hyper-contractile heart and is the most common cause of sudden cardiac arrest in people under 35 years old. Based on insight from a dream, Spudich realized that many of the mutations associated with HCM are in a region of the myosin molecule (the myosin mesa) that may regulate the availability of myosin heads to bind to actin and thus, regulate muscle contraction. Spudich’s lab is now working to determine the importance of the myosin mesa in regulating cardiac contractility and, in particular, its role in HCM.
Speaker Bio
James Spudich

James Spudich is the Douglass M. and Nola Leishman Professor of Cardiovascular Disease in the Department of Biochemistry at Stanford University School of Medicine. For the past several decades, his lab has studied the structure and function of the myosin family of motor proteins. More recently Spudich’s lab has focused on human cardiac muscle myosin… Continue Reading








Leave a Reply