Talk Overview
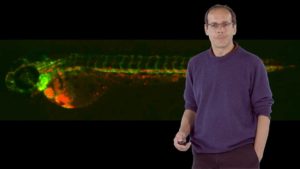
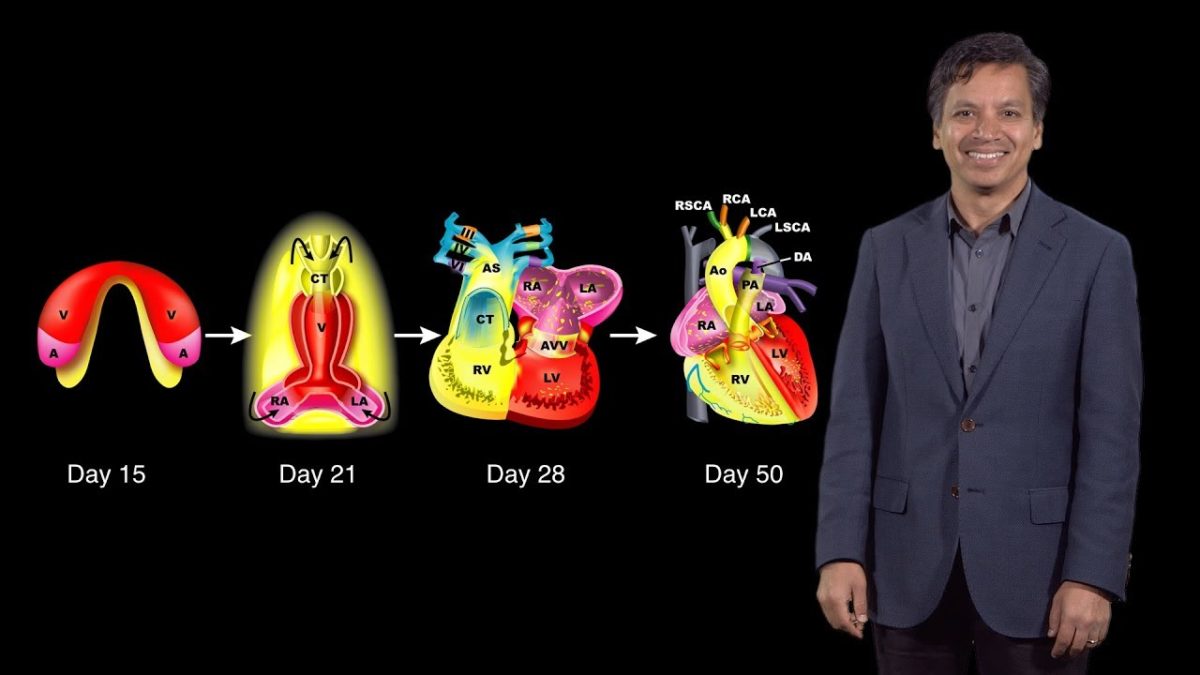
How does a fertilized egg develop into a complex multicellular organism such as a fly, mouse or human? During zebrafish heart development, for example, cells must proliferate, differentiate, move and come together to form a complex organ. Didier Stainier explains that zebrafish are an excellent model organism in which to address this question because their eggs are externally fertilized, they produce many offspring and the embryos and larvae are translucent. In addition, specific cells can be fluorescently labelled making it easier to image organ development in live fish. Taking advantage of these characteristics, Stainier and his colleagues performed large forward genetic screens to look for mutants in zebrafish heart development. Their findings provide insight into the evolution and development of the vertebrate heart.
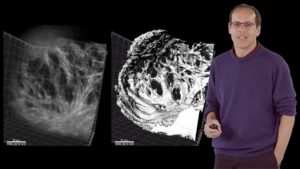
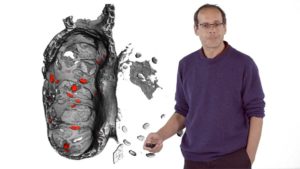
In his second lecture, Stainier describes work from his lab investigating the formation of trabeculae in zebrafish hearts. Trabeculae are multicellular protrusions into the lumen of the ventricle that allow the heart to increase in muscle mass and thus pump more forcefully. Interestingly, trabeculae form only in the ventricle, not in the atrium, and only on the outer curvature of the ventricular lumen. For trabeculae to form, cardiomyocytes must delaminate from the outer layer of muscle cells and proliferate in the lumen. Stainier discusses how his lab identified factors regulating this process including the important roles of blood flow and contractility.
Gene function in zebrafish has been investigated by 1) randomly mutagenizing the genome, 2) knocking down genes with antisense oligos or 3) more recently, by specifically mutating a gene of interest with gene editing tools. Interestingly, phenotypes obtained by antisense knockdown are often more severe or different than those obtained by gene knockout. In his last lecture, Stainier presents work from his lab that compares knockdown vs knockout of the egfl7 gene in zebrafish (causing severe vs mild vascular defects) and asks why this difference in phenotypes occurs. He walks us through the experiments which show that in the case of egfl7, and numerous other genes, gene knockout effects are compensated by upregulated transcription of paralogous or related genes. This finding raises many questions about how this phenomenon occurs and Stainier’s group continues to investigate this and related questions.
Speaker Bio
Didier Stainier

Dr. Didier Stainier is a Director (Principal Investigator) in the Department of Developmental Genetics at the Max Planck Institute for Heart and Lung Research (MPI-HLR), in Bad Nauheim, Germany. His lab uses the zebrafish as a model to study development of the cardiovascular system and pancreas, and the mouse as a model for lung development…. Continue Reading






Eamon says
Fantastic works!