Talk Overview
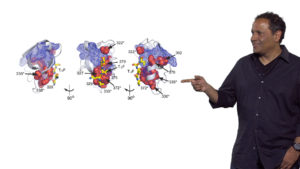
Proteins are synthesized as linear polymers of amino acids, yet proteins spontaneously fold into complex 3 dimensional structures, fulfill biochemical functions, and are able to adapt to a changing environment. How does this “protein design” happen? In Part 1, Ranganathan explains that the transformation is directed by physical interactions between small numbers of amino acids within a protein. Most interactions are short-range but some are surprisingly long-range and interactions between amino acids in the active sites of proteins may be co-operative. Amino acids can also cluster in modules allowing different parts of a protein to have different functions.
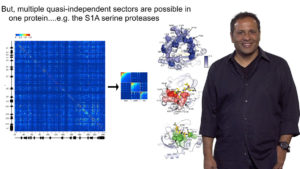
In Part 2, Ranganathan describes a statistical approach developed by his lab to determine which amino acids in a protein drive the “design” of that protein. By studying the same protein across many species, Ranganathan and colleagues determined which amino acids were highly conserved, and thus likely important for protein function. They also tracked pairs or groups of residues where variation in one residue resulted in a change in the other residue, indicating conserved interactions. These experiments gave rise to a model in which a few, physically connected, collectively evolving groups or “sectors” of amino acids provide the “design” for natural proteins.
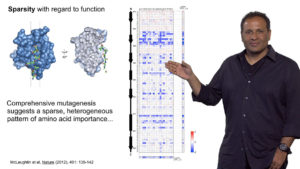
In his third lecture, Ranganathan describes experiments done in his lab to test the model proposed in Part 2. They find that a statistical matrix that predicts interactions between amino acids provides sufficient information to encode protein structure. Using saturation mutagenesis, Ranganathan’s lab showed that amino acids necessary for a protein function, such as ligand binding, reside within a sector. Using a similar technique, Ranganathan was able to determine which amino acids in a protein were most likely to mutate or adapt in response to selective pressure. Again, all of the amino acids identified were in a sector position. These findings support a model in which sectors provide the necessary design parameters for protein folding, function and adaptability.
Speaker Bio
Rama Ranganathan

Rama Ranganathan received his B.S. in Bioengineering from the University of California, Berkeley and his Ph.D. and M.D. degrees from UC San Diego where he studied signal transduction in the invertebrate visual system. He was a post-doctoral fellow at Harvard Medical School, where he studied K+ channels, and at The Salk Institute, where he learned… Continue Reading





Leave a Reply