Talk Overview
Horwich begins with a brief history of the discovery of the chaperonins and their importance in proper protein folding. He then gives an overview of two well-studied families of chaperones, heat shock protein 60 (Hsp60) and Hsp70.
In Part 1 B, Horwich continues with a review of the Calnexin, Hsp90 and small Hsp families. He discusses how transcriptional regulation in response to chemical or heat stress results in an increase in effector chaperonins. Finally, Horwich poses the question of why chaperonins are not effective in preventing protein aggregation or misfolding in a number of devastating neuronal diseases.
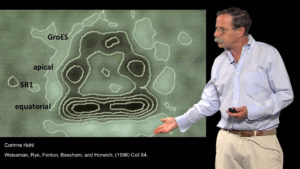
Horwich discusses experiments designed to decipher how chaperones mediate protein folding in Part 2 of his talk. He describes in detail the role of ATP binding and hydrolysis by the GroEL/GroES complex in promoting correct protein folding.
In Part 3, Horwich describes the experiments that determined that proteins are, in fact, folded within the chaperonin complex and exactly where folding occurs.
Speaker Bio
Arthur Horwich

Arthur Horwich received his AB and MD from Brown University. Following a pediatric residency at Yale, Horwich did two postdoctoral fellowships, first with Tony Hunter and Walter Eckhart at the Salk Institute and second with Leon Rosenberg at Yale, before joining the Yale faculty. Currently, Horwich is Sterling Professor of Genetics and Pediatrics at Yale… Continue Reading







Leave a Reply